Contents
Molecular Nature of an Atom
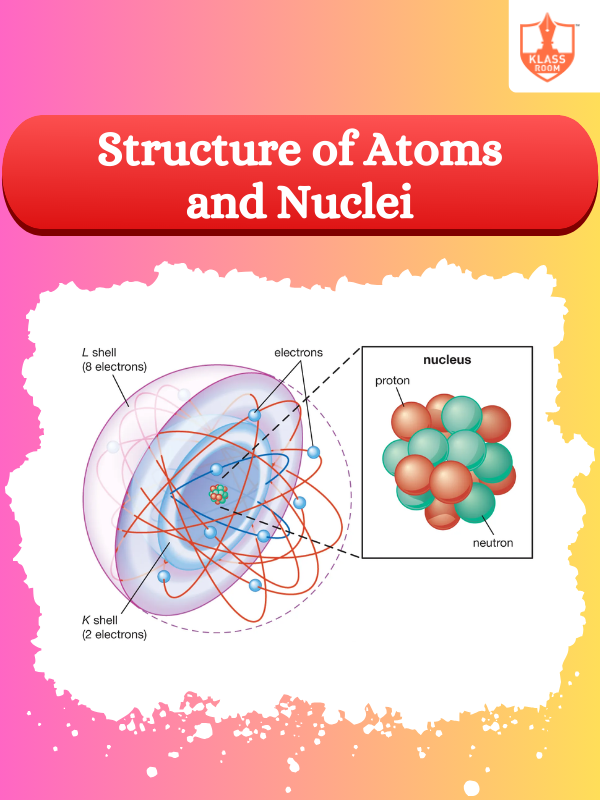
Description: Atoms consist of a nucleus (protons and neutrons) surrounded by electrons, determining chemical properties through interactions and bonding mechanisms.
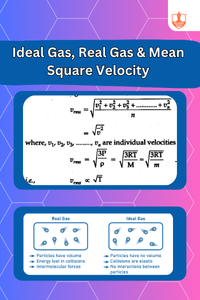
Ideal Gas, Real Gas & Mean Square Velocity
Description: Avogadro's Hypothesis states equal volumes of gases have equal molecules. Ideal gases follow gas laws; real gases deviate due to interactions.
.png)
Gas Law's
Description: Gas laws describe relationships between pressure, volume, temperature, and amount of gas molecules in ideal gas behavior.
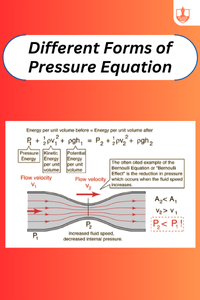
Different Forms of Pressure Equation
Description: Pressure equations quantify forces exerted on surfaces or within fluids, crucial in physics, engineering, and fluid dynamics for analysis and design.

Types of Motion & Degrees of Freedom
Description: Types of motion include translational, rotational, and oscillatory. Degrees of freedom are independent parameters defining an object's spatial movements.

Specific Heat Capacity & Mean Free Path
Description: Specific heat capacity is energy required to raise temperature; mean free path is average distance between collisions in gases.