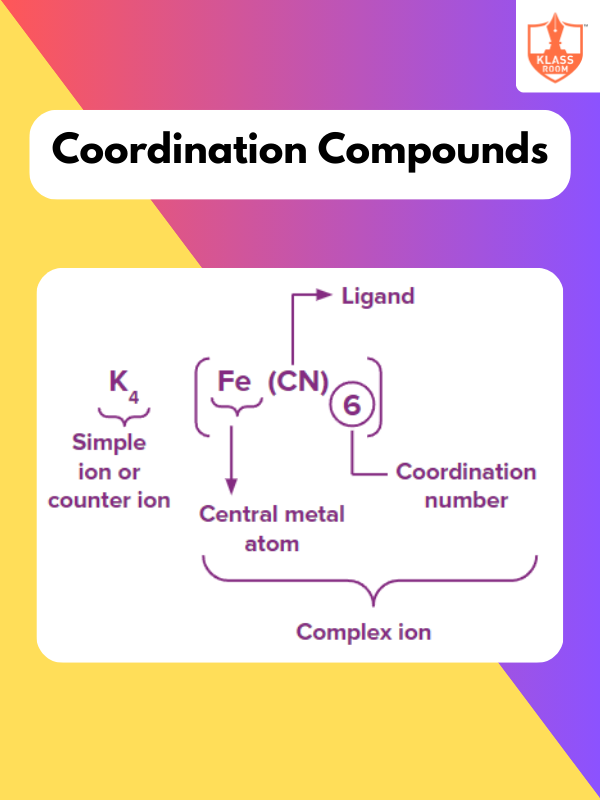
Contents
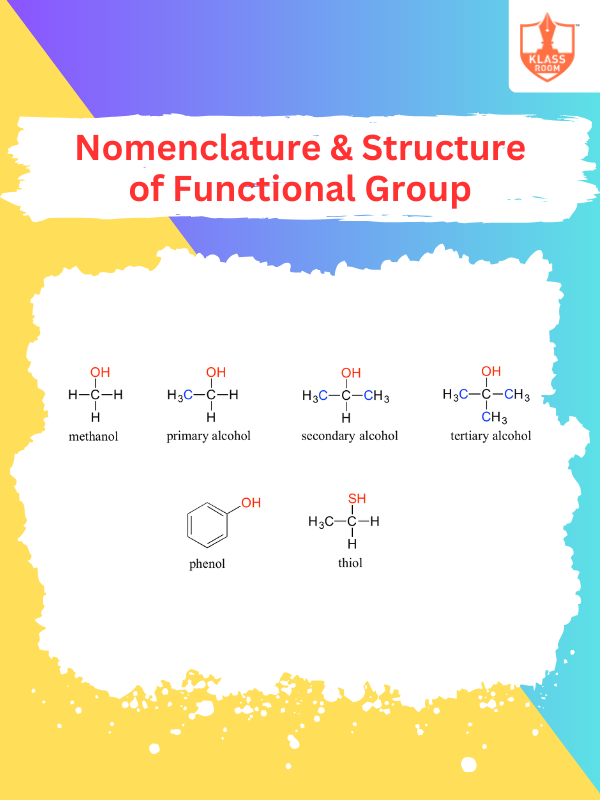
Nomenclature & Structure of Functional Group
Description: Functional groups are named based on priority, and their structure defines chemical properties and reactivity.

Alcohol & Its Preparation
Description: Alcohols are organic compounds with -OH group, prepared via hydration, reduction, and fermentation methods.
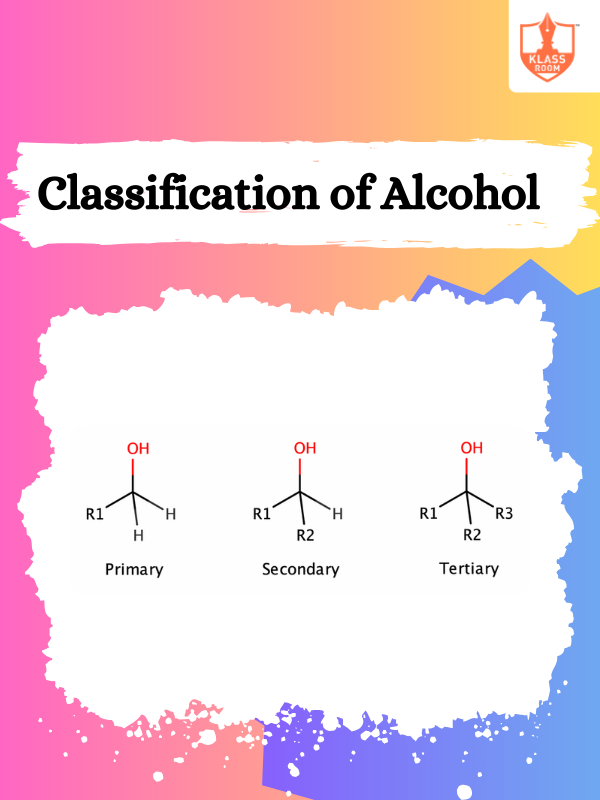
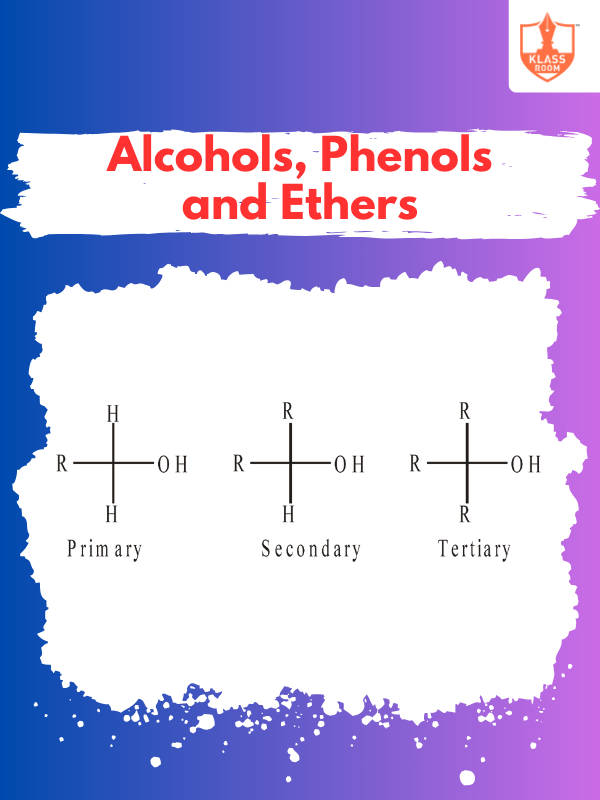
Classification of Alcohol
Description: Alcohol is classified into primary, secondary, and tertiary based on the carbon atom bonded to hydroxyl.
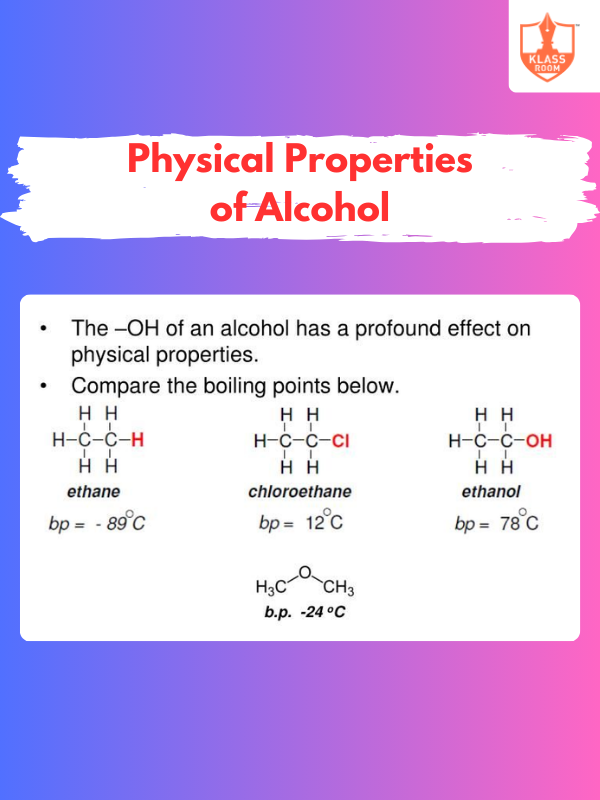
Physical Properties of Alcohol
Description: Alcohols have high boiling points, hydrogen bonding, are polar, soluble in water, and can evaporate easily.
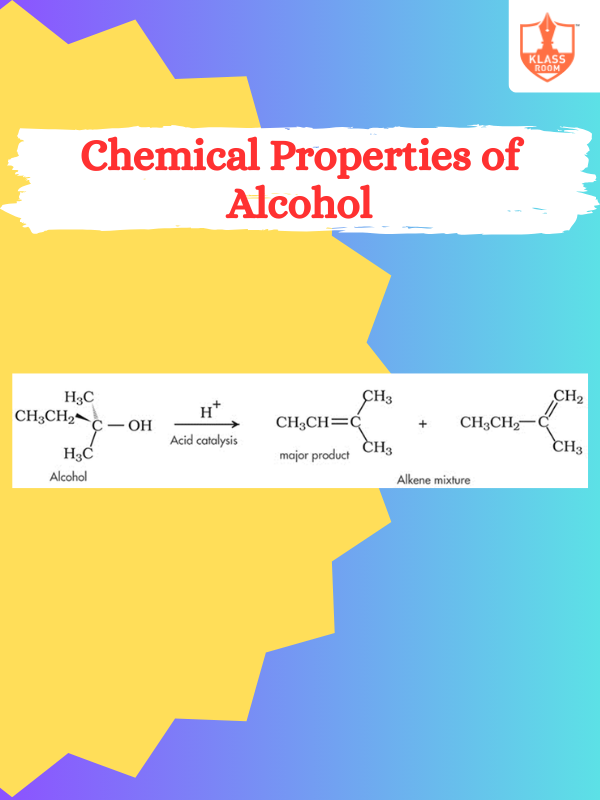
Chemical Properties of Alcohol
Description: Alcohols undergo oxidation, esterification, dehydration, nucleophilic substitution, combustion, and react with metals to form alkoxides.
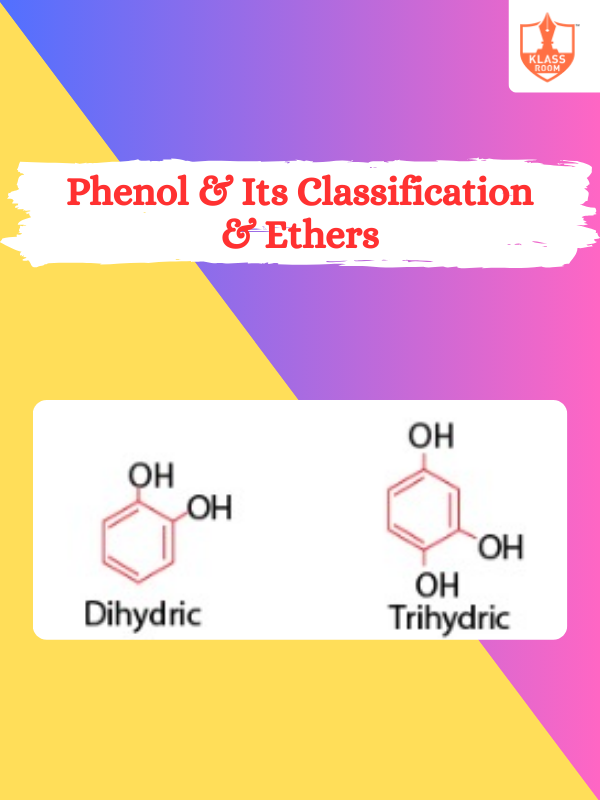
Phenol & Its Classification & Ethers
Description: Phenol is an aromatic alcohol, acidic, antiseptic, classified by substituents; ethers are organic compounds with -O- linkage.
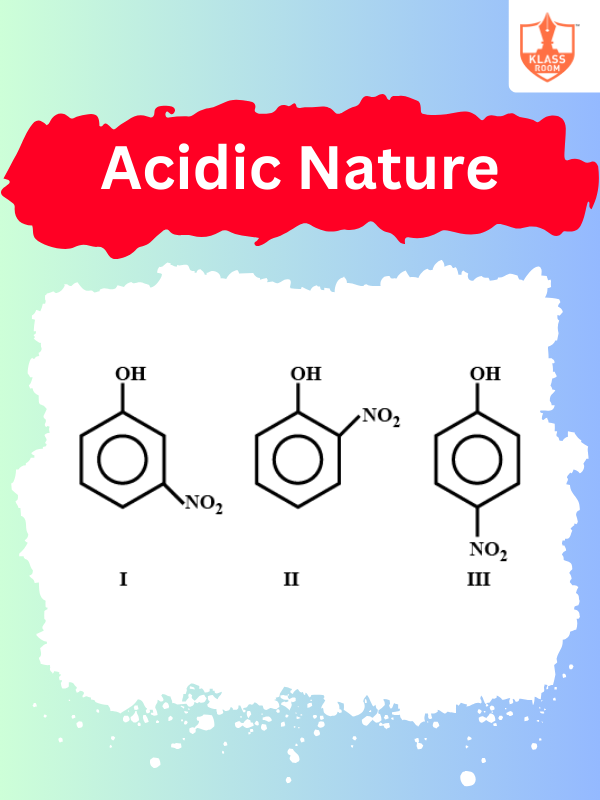
Acidic Nature
Description: Acidic nature refers to a substance's ability to donate protons (H⁺) and lower pH levels.

Physical & Chemical Properties of Ethers
Description: Ethers are volatile, low polarity, insoluble in water, flammable, unreactive to acids, form peroxides easily.


.png)