Contents
Amines & Structure of Amines
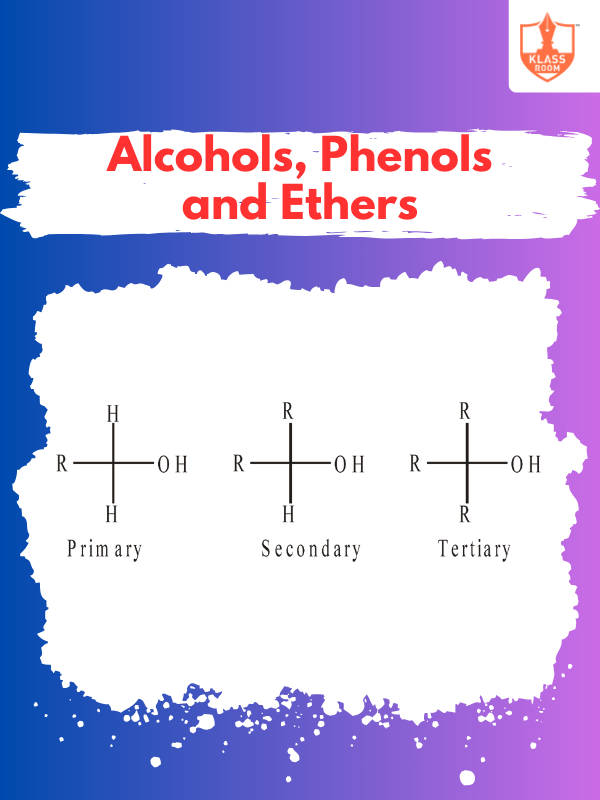
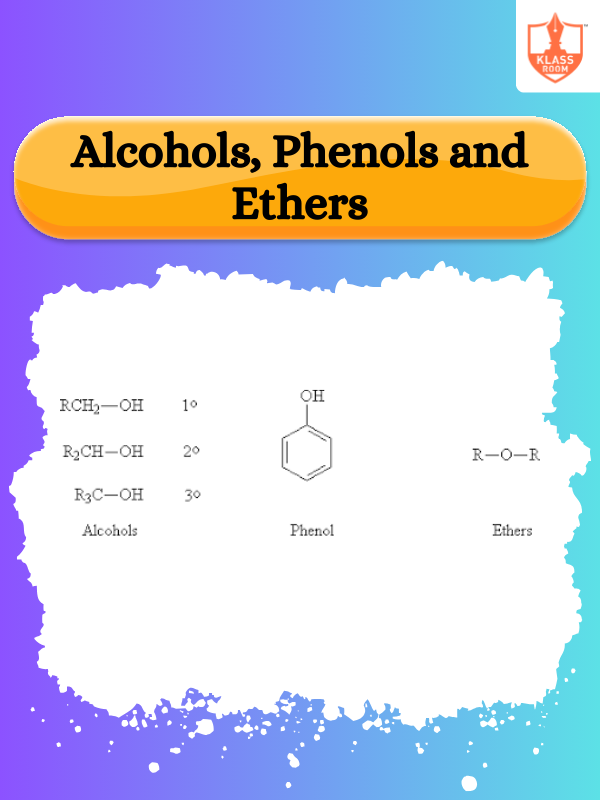
Description: Amines are ammonia-derived compounds with nitrogen bonded to hydrogen or carbon, classified as primary, secondary, or tertiary.
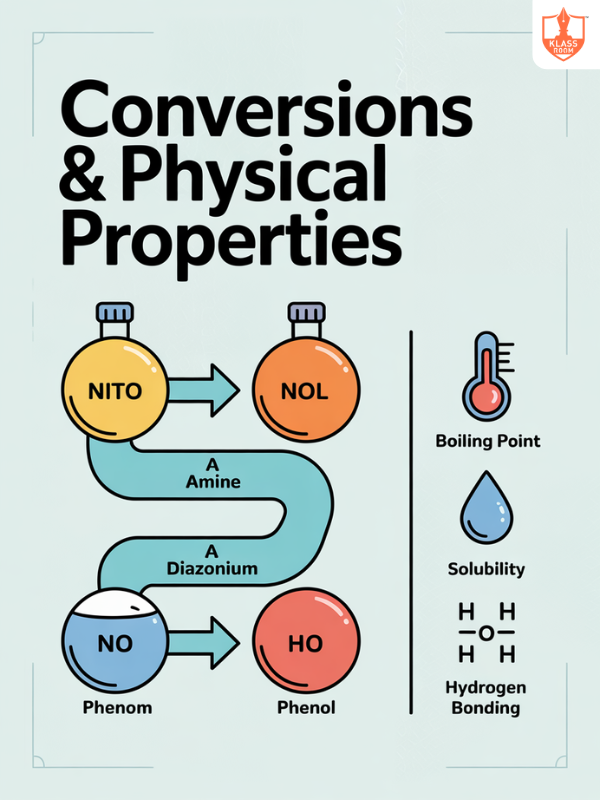
Conversions & Physical Properties
Description: Conversions of amines include alkylation, acylation, and diazotization, while physical properties depend on hydrogen bonding and polarity.
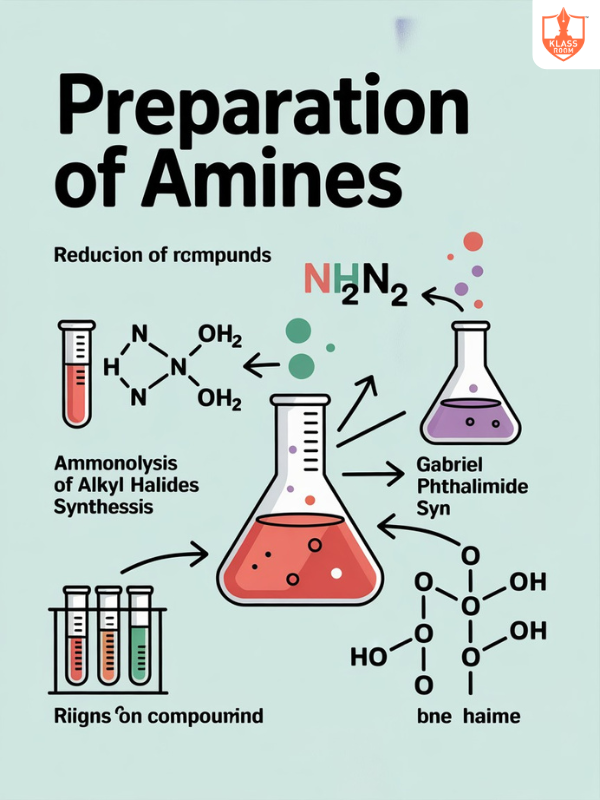
Preparation of Amines
Description: Amines are prepared by reduction of nitro compounds, amides, nitriles, reductive amination, and Gabriel phthalimide synthesis.
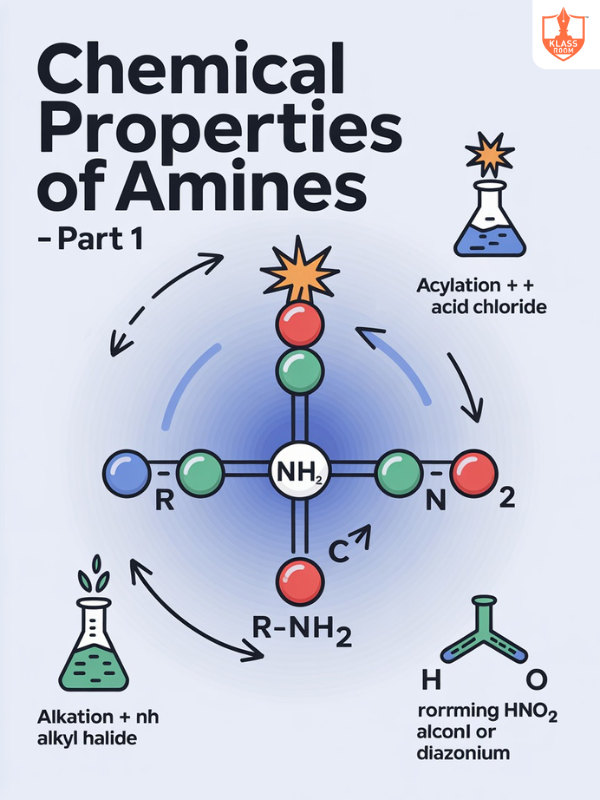
Chemical Properties of Amines Part-1
Description: Chemical properties of amines include basicity, nucleophilic substitution, alkylation, acylation, oxidation, and reaction with nitrous acid.
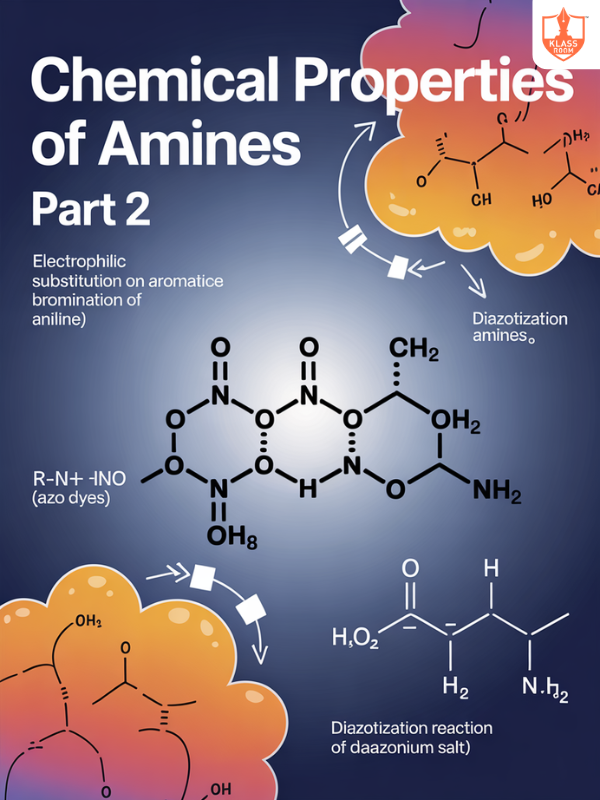
Chemical Properties of Amines Part-2
Description: Chemical properties of amines include basicity, salt formation, acylation, alkylation, oxidation, and reaction with nitrous acid.

Electrophilic Substitution of Amines
Description: Electrophilic substitution of amines includes bromination, nitration, and sulfonation, occurring at ortho and para positions.
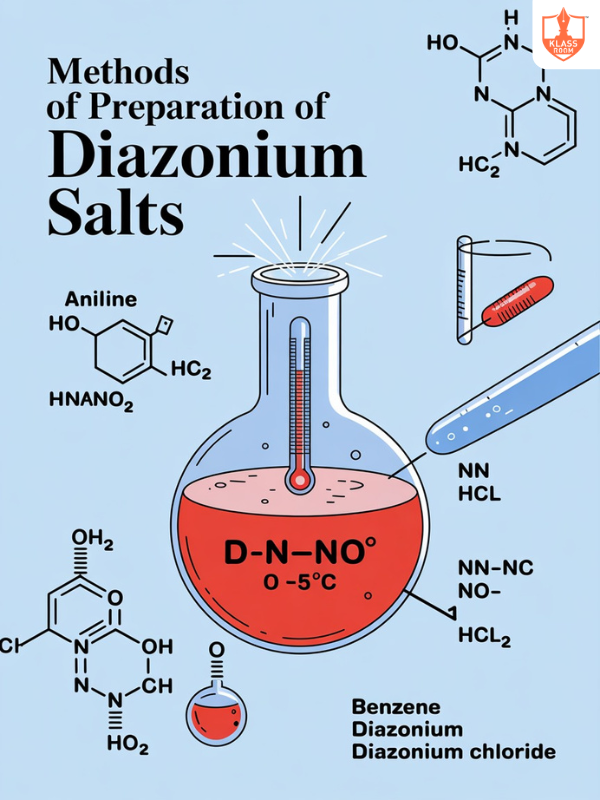
Methods of Preparation of Diazonium Salts
Description: Diazonium salts are prepared by reacting primary aromatic amines with sodium nitrite and hydrochloric acid at low temperatures.
Introduction of Cyanides & Isocyanides
Description: Cyanides (-CN) and isocyanides (-NC) are nitrogen-containing organic compounds with different bonding, structures, and chemical reactivities.
Gabriel Phthalimide Synthesis
Description: Gabriel phthalimide synthesis produces primary amines using phthalimide, alkyl halide, and hydrolysis in acidic or basic medium.
.png)