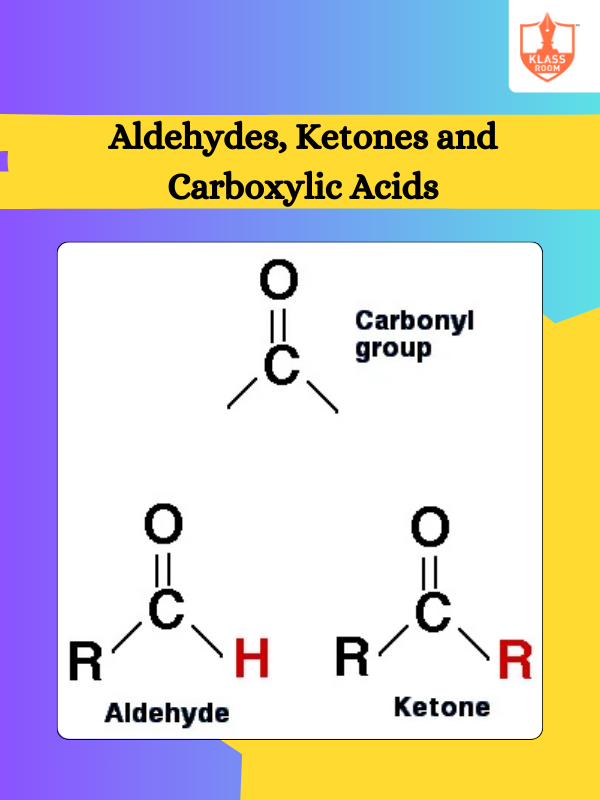
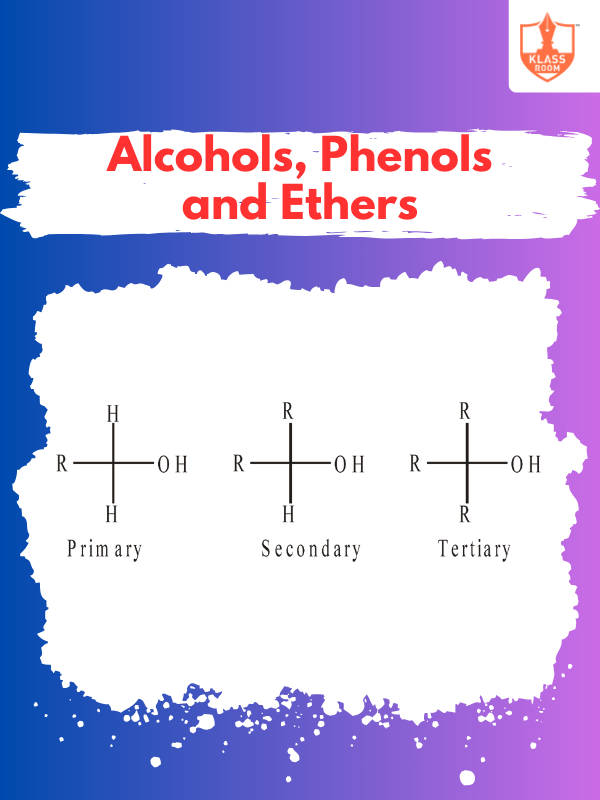
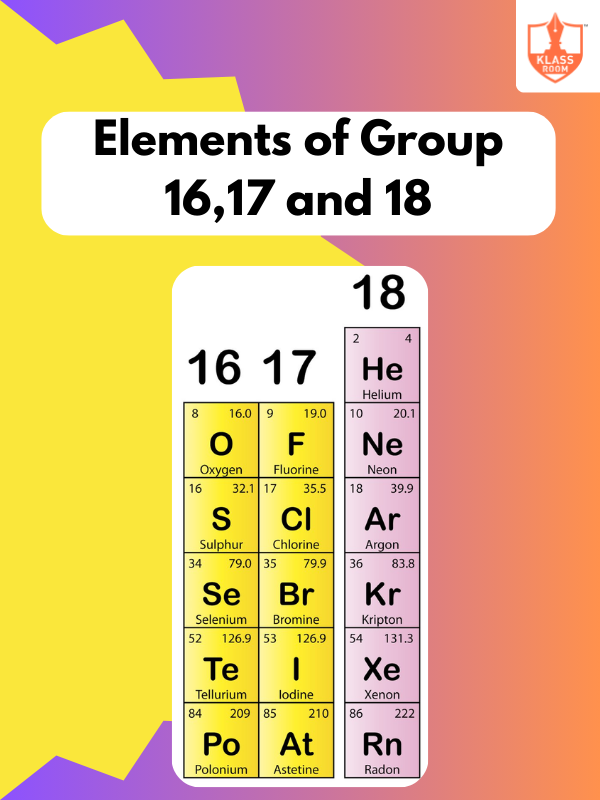
Contents
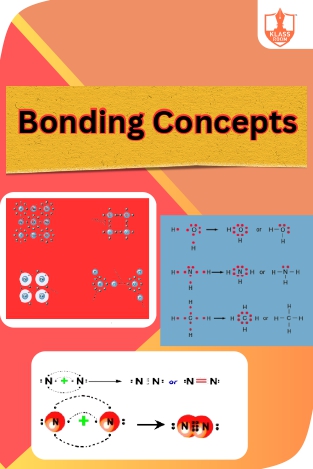
Bonding Concepts
Description: Chemical Bonding encompasses types like ionic and covalent bonds, Theories elucidate molecule formation, and Lewis Symbols depict electron arrangement via dot structures.
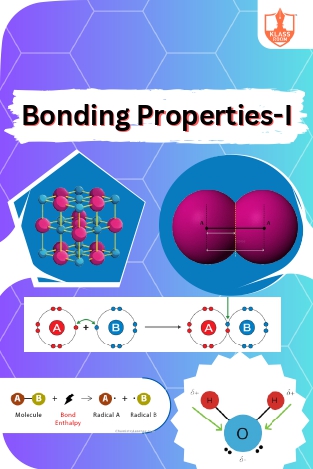
Bonding Properties-I
Description: Covalent and ionic bonds differ in electron sharing; Ionic compounds possess unique properties; Bond parameters and enthalpy measure bond strength; Resonance and polarity influence molecular behavior.

Bonding Properties-II
Description: Fajan’s Rules describe polar covalent bonding; VSEPR predicts molecular geometry, with its applications and limitations; Quantum Mechanics explains electron behavior; Sigma and pi bonds influence molecular structure.
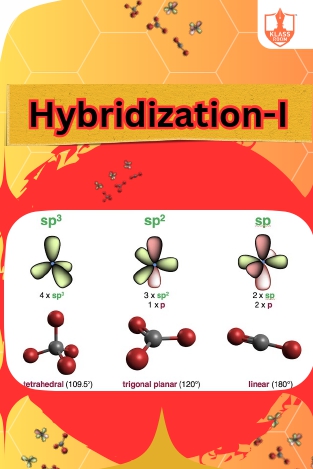
Hybridization-I
Description: Hybridization explains atomic orbitals' mixing to form new orbitals, labeled sp, sp2, sp3, and sp3d, influencing molecular geometry and bonding characteristics.
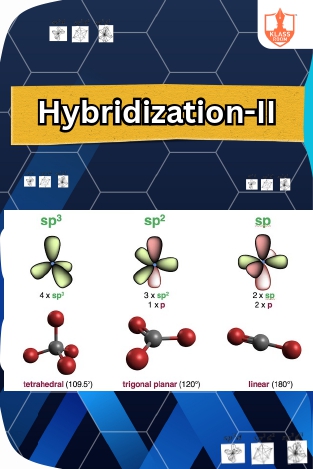
Hybridization-II
Description: sp3d2 and sp3d3 hybridization involve the mixing of s, p, and d orbitals to form new hybrid orbitals, found in certain molecules with expanded octets.
Breathing and Exchange of Gases
Description: Breathing involves inhaling oxygen and exhaling carbon dioxide, facilitated by the lungs through diffusion between air sacs and blood vessels.

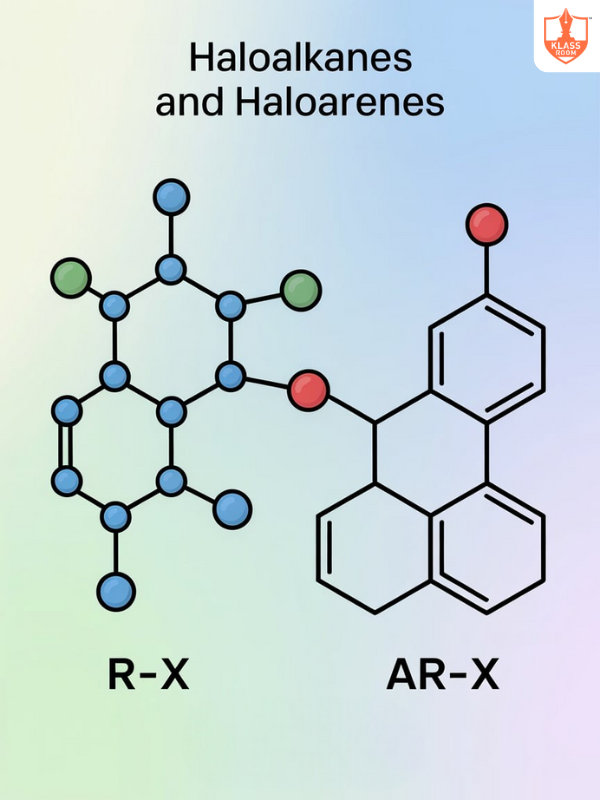

.png)