Contents
,_Liquid-Vapour_or_Gas_Equilibrium_-_(I_&_II)_.jpg)
Equilibrium-I,II & III
Description: Introduction to Equilibrium covers static and dynamic equilibrium, including Le Chatelier's Principle. Liquid-vapor or gas equilibrium explores phase transitions and factors influencing vapor pressure in closed and open systems.

Equilibrium Law
Description: Solid dissolution, gas-liquid equilibrium, dynamic chemical equilibria, law defining equilibrium conditions, and constant characterizing equilibrium states are fundamental concepts in chemistry.
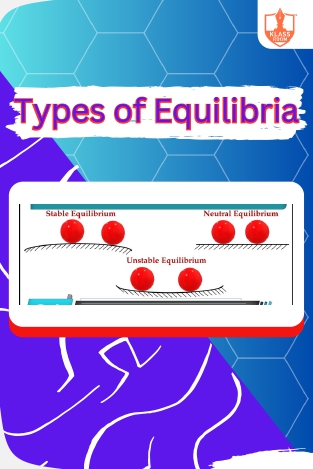
Equilibria
Description: "Types of Equilibria" encompasses static, dynamic, and chemical equilibrium, crucial concepts in understanding balance and stability in various systems.
,_Calculating_the_Equilibrium_Concentrations_.jpg)
Equilibrium-Calculating
Description: "Equilibrium Constant and Multiples, Predicting Reaction Extent (I & II), Calculating Equilibrium Concentrations" involves quantifying reactions and predicting their outcomes.

K & Q & G
Description: "Relationship Between Equilibrium Constant, Reaction Quotient, and Free Energy involves thermodynamic principles governing reversible reactions and their equilibrium states."
_.jpg)
Gaseous Equilibrium
Description: "Pressure effects on gaseous equilibrium, inert gas addition, NO2 dimerization, Le Chatelier's Principle applications, and introduction to acidic and basic nature."

Acids in Industry
Description: "Acid properties include corrosiveness and sour taste. Mineral acids find application in industries such as chemical manufacturing, metallurgy, and wastewater treatment."
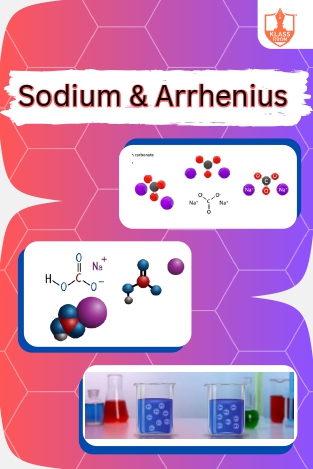
Sodium & Arrhenius
Description: "Sodium bicarbonate and carbonate are alkaline compounds. Arrhenius proposed acids release hydrogen ions, bases release hydroxide ions when dissolved in water."
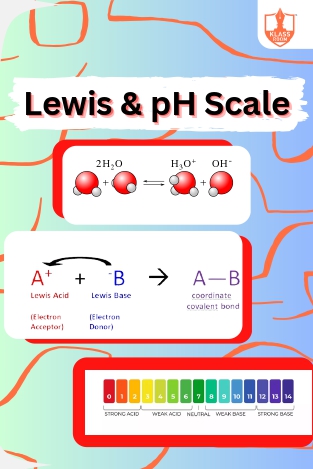
Lewis & pH Scale
Description: "Lewis theory defines acids as electron pair acceptors, bases as donors. The ionization constant of water (Kw) relates to pH scale, measuring acidity or alkalinity."
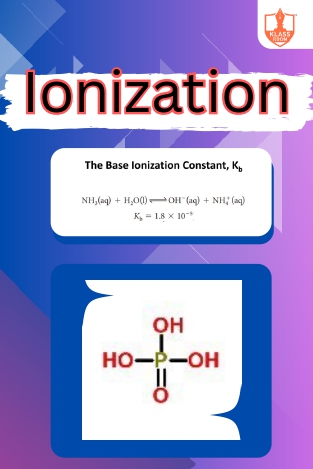
Ionization
Description: "Ionization constant of weak bases quantifies their strength. Polybasic acids donate multiple protons. Problems based on ionization explore equilibrium calculations in acidic solutions."

Problems
Description: Problems Based on Ionization-II Solution

Solubility
Description: "Solubility equilibria explores sparingly soluble salts. Classification categorizes salts by solubility. Solutions types vary by solute amount. Solubility product quantifies salt solubility."


.png)