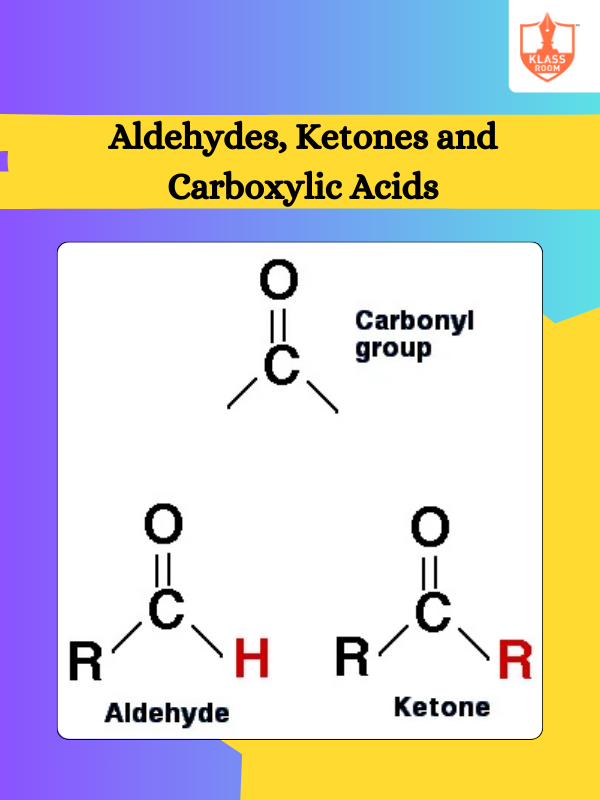
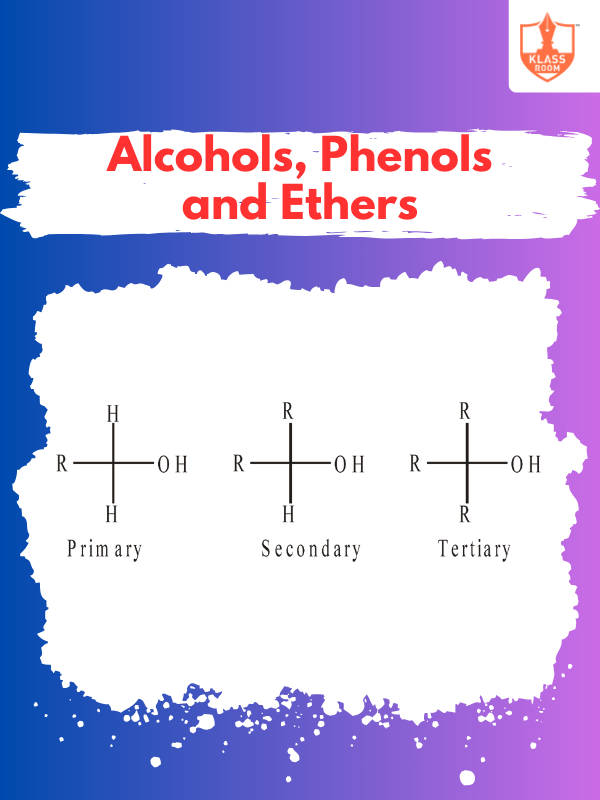
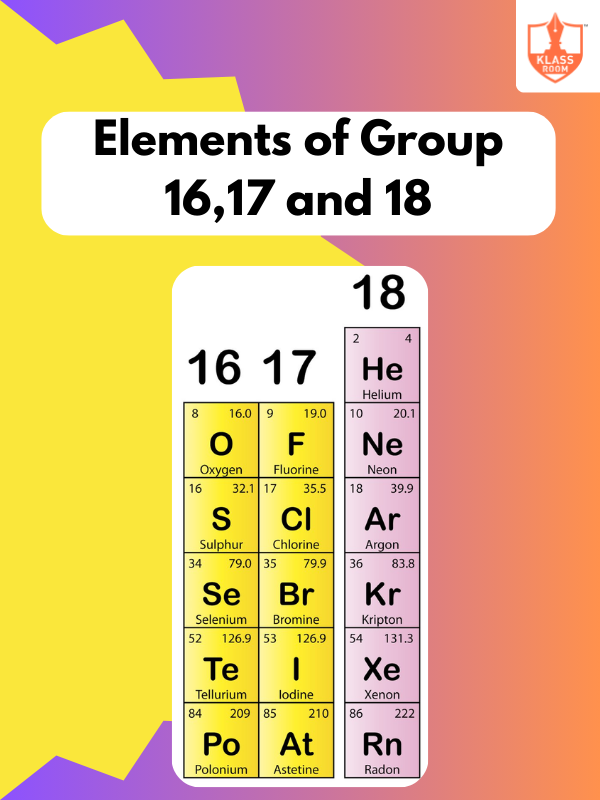
Contents
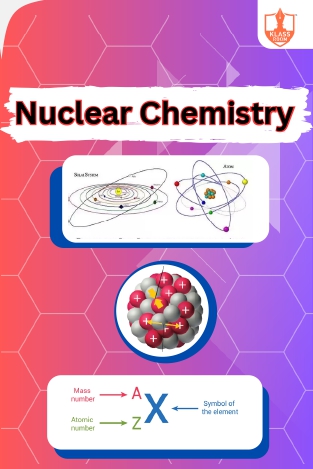
Nuclear chemistry
Description: "Introduction to nuclear chemistry explores atomic structure and similarities with the solar system. Concepts include mass number, atomic number, nuclide classification, and nuclear stability criteria."
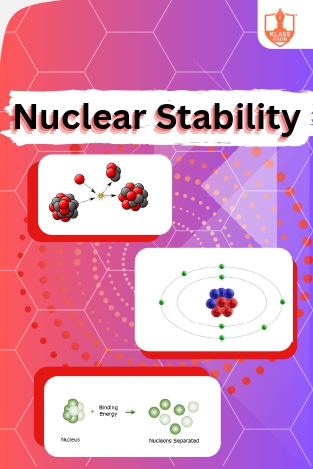
Nuclear stability
Description: "Nuclear stability concerns even-odd proton-neutron counts and neutron-proton ratios (N/Z). Magic numbers denote stable nuclei configurations. Nuclear potential, binding energy, mass defect, and radioactivity elucidate nuclear phenomena."

Radioactive decay
Description: "Radioactive decay follows rate laws with decay constants. Decay constant expressions relate to decay rates. Half-life (t1/2) measures time for half the radioelement to decay."
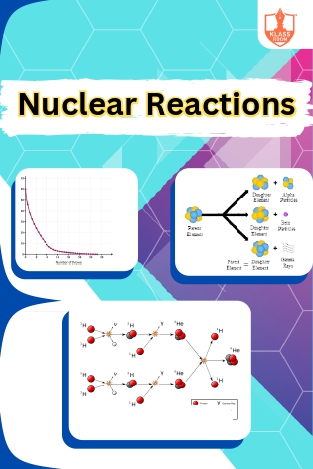
Nuclear Reactions
Description: "Decay is graphically represented to illustrate activity changes over time. Units of radioactivity measure decay rates. Modes of decay include alpha, beta, and gamma emissions. Nuclear reactions involve transmutation and induced radioactivity, including f
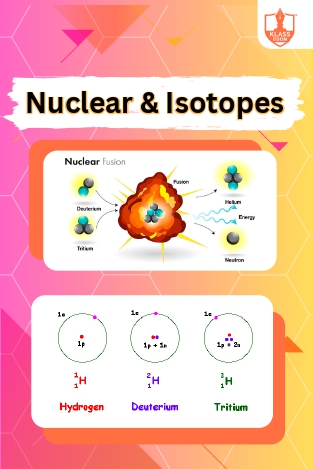
Nuclear & Isotopes
Description: "Nuclear fusion involves combining nuclei to release energy. Applications of radioisotopes include medical diagnostics, industrial processes, and agriculture, benefiting various fields with their unique properties and capabilities."
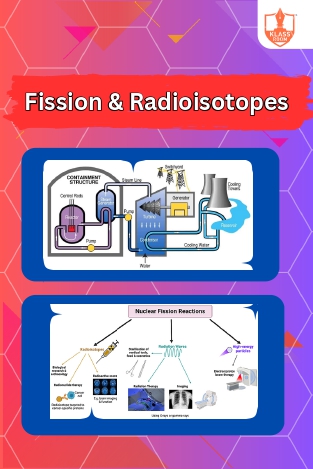
fission & Radioisotopes
Description: "Nuclear fission generates electrical energy through controlled splitting of atoms. Radioisotopes find medical applications in diagnosis and therapy, as well as in various fields for sterilization, dating, and material analysis."


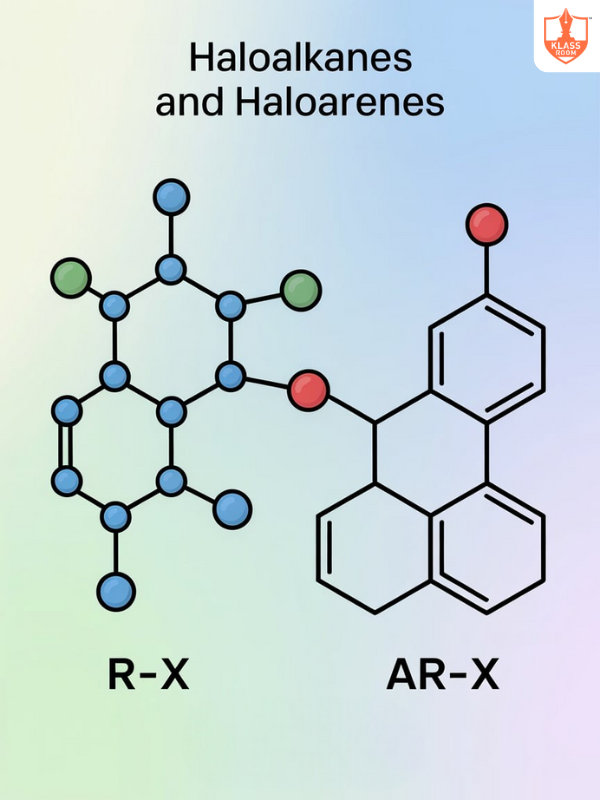

.png)