Contents

Solutions
Description: Solutions are homogeneous mixtures of solutes and solvents, with properties like concentration, solubility, and colligative effects.

Description: Solutions are homogeneous mixtures of solutes and solvents, with properties like concentration, solubility, and colligative effects.

Board: State Board
Stream: Science
Standard: XI
Course: Chemistry
Know More.png)



Board: State Board
Stream: Science
Standard: XI
Course: Chemistry
Know More
Board: State Board
Stream: Science
Standard: XI
Course: Chemistry
Know More
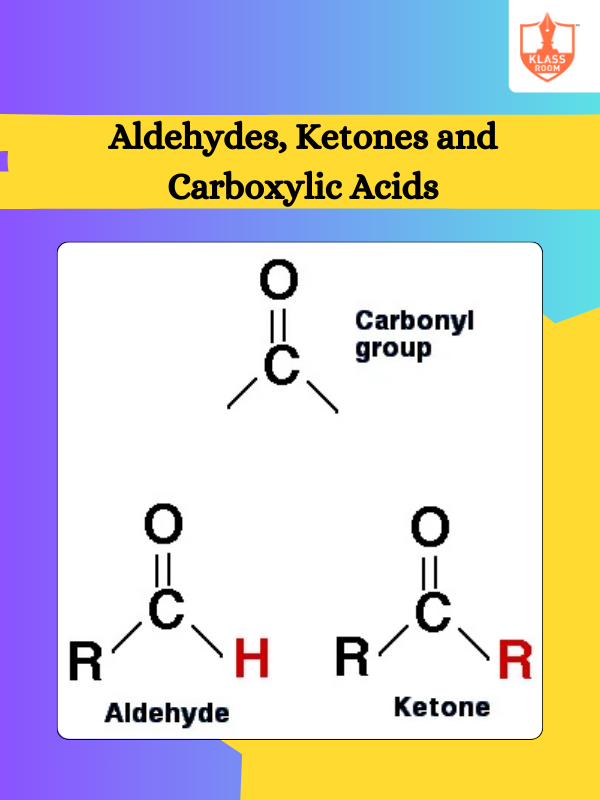
Board: State Board
Stream: Science
Standard: XII
Course: Chemistry
Know More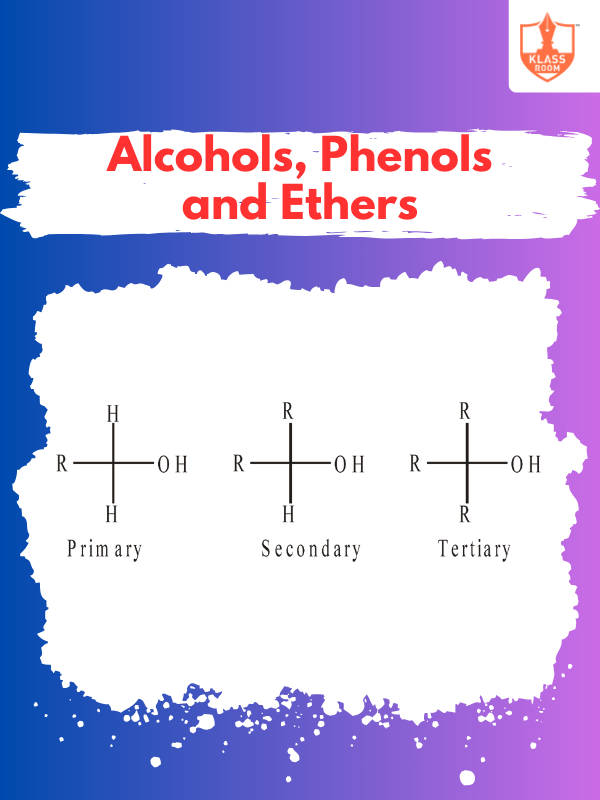
Board: State Board
Stream: Science
Standard: XII
Course: Chemistry
Know More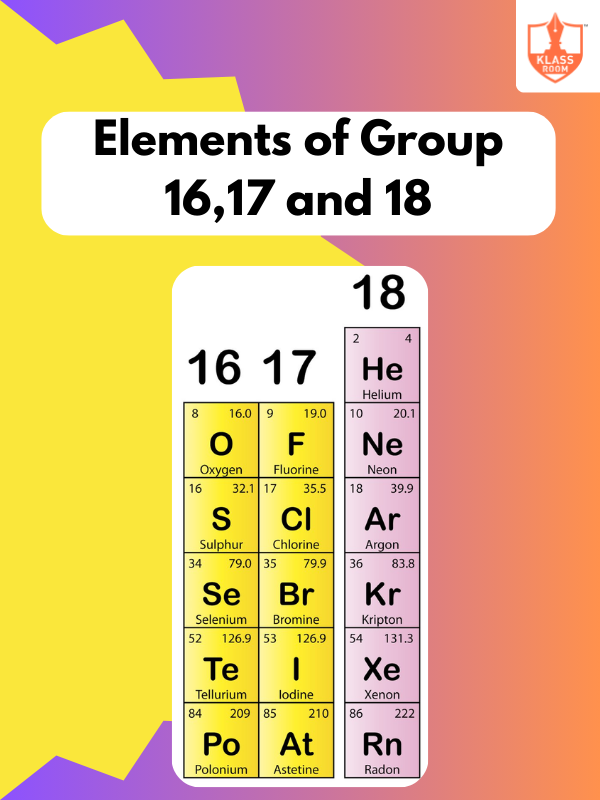
Board: State Board
Stream: Science
Standard: XII
Course: Chemistry
Know More